A Compound That Produces Hydroxide Ions When Dissolved in Water
Bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Water glass also called sodium silicate or soluble glass a compound containing sodium oxide Na2O and silica silicon dioxide SiO2 that forms a glassy solid with the very useful property of being soluble in water.

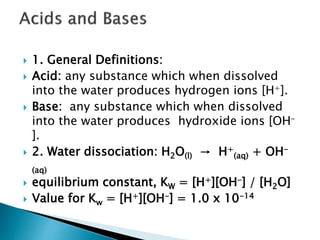
Arrhenius Definition Of Acids Bases Substances That Produce Hydrogen Ions H When Dissolved In Water Substances That Produce Hydroxide Ions Oh Ppt Download
When dissolved in water the strong base sodium hydroxide ionizes into hydroxide and sodium ions.

. Acid is a chemical compound that gives rise to a solution with ionic activity greater than that of pure water when dissolved in water. -oxidation-reduction and synthesis -oxidation-reduction only -synthesis. NaOH Na OH and similarly in water the acid hydrogen chloride forms hydronium and chloride ions.
All magnesium ions precipitate as magnesium hydroxide before the indicator is added. The Patton-Reeder indicator is a suitable indicator in this case as it produces a clear colour change from pinkred to blue in the pH range of 12 14. Water glass is sold as solid lumps or powders or as a clear syrupy liquid.
HCl H 2 O H 3 O Cl When the two solutions are mixed the H. The fundamental concept of this theory is that when an acid and a base react with each other the acid forms its conjugate base and the base forms its conjugate acid. -NaCl - CaCl2 -FeCl3 -BaCl2 - PbCl2 The equation 2Als 3Br2l 2AlBr3s is an _____ reaction.
In aqueous solutions a base gives off electrons accepts protons or releases hydroxide ions OH -. Memorize flashcards and build a practice test to quiz yourself before your exam. Start studying the chem quiz 7 flashcards containing study terms like Which of the following ionic compounds is insoluble in water.
It is used as a convenient source of sodium for many industrial products as a builder in laundry. The BrønstedLowry theory also called proton theory of acids and bases is an acidbase reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The pH will be approximately 125 due to the addition of concentrated NaOH solution.

Acids And Bases Chemistry Notes Chemistry Basics Chemistry Education

Chloe Mediocre 16 Year Old Singaporean O Level Student Aiming For Mass Comms Est 10 02 16 My Science Notes Chemistry Notes Teaching Chemistry

Organic Archives Practically Science In 2021 Organic Chemistry Study Chemistry Lessons Chemistry Education

Chapter 14 15 1 Acids Bases And Ph Ppt Download

Studyboba Study Notes Study Organization Pretty Notes

Acids And Bases Acids Bases Acids Are Substances That Produce Hydrogen Ion In Solution H Aq Bases Are Substances That Produce Hydroxide Ions Ppt Download

Introduction To Atomic Theory Educational Infographics For Chemistry Video Atomic Theory Organic Chemistry Chemistry
What Releases Hydroxide Ions In A Water Solution Quora

Acids And Bases An Acid Is A Substance That Produces A Hydronium Ion H 3 O When Dissolved In Water Acid Nearly All Acid Molecules Contain One Or Ppt Download

Acids Bases And Salts Ppt Download

Acids Any Substance That Produces H When Dissolved In Water 2 Types Binary Hydrogen And One Other Element Oxyacids Contains Hydrogen And Oxygen Ppt Download

Chart Acids And Bases Chemistry Lessons Chemistry Basics Teaching Chemistry

One Pagers Not Just For Ela Anymore Middle School Science Classroom Middle School Science Teacher Science Classroom





Comments
Post a Comment